The latest report released by the World Health Organization (WHO) stated that up to 10% of medicines on the global market are substandard or falsified medicines that are a main source of public health threat. The alarmingly high number highlights the urgent need for stronger pharmaceutical packaging security solutions.
These solutions are the first line of defense against the counterfeiting of drugs. They provide tamper-evident protection while meeting strict regulatory compliance. In this article, we will discuss FDA security solutions, such as tape and labels, and why they are essential at every stage of drug distribution in the pharmaceutical industry.
Understanding FDA Requirements for Pharmaceutical Packaging
The Food and Drug Administration sets requirements for pharmaceutical packaging under the Food, Drug, and Cosmetic Act. Unlike generic packaging solutions, FDA-approved packaging options must meet strict FDA compliance requirements. These rules focus on tamper-evident features that protect patients and preserve product integrity during transport and storage.
Packaging (including both tapes and labels) for prescription and over-the-counter drugs must show clear evidence if opened. This helps reduce risks of contamination. It also lowers the chance of counterfeiting.
So, labels and seals are a key part of meeting compliance. Using FDA (Food, Drug, and Cosmetic Act)-approved solutions shows that a company has taken the right steps to safeguard products.
Also, clear records of packaging methods, materials, and inspection results help demonstrate accountability and readiness during audits. Manufacturers that comply not only meet legal obligations but also safeguard public health and strengthen brand confidence
FDA Security Tapes: A First Line of Defense
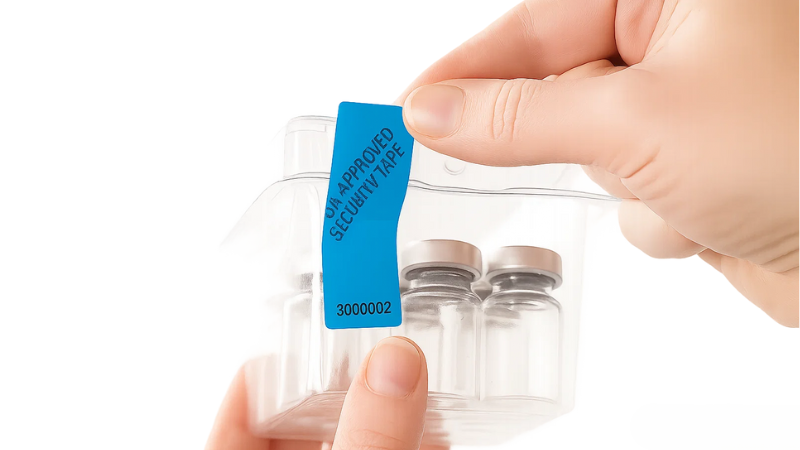
FDA Security Tape is the type of security tape designed to meet strict pharmaceutical packaging standards. It provides a visible layer of protection by showing clear signs if a package has been opened or altered. This makes it harder for counterfeit or contaminated drugs to reach patients.
- Commonly applied on pill bottles, blister packs, shipping cartons, and clinical trial kits
- Available in different adhesive strengths to suit varied packaging
- Can withstand shifts in temperature and humidity during storage and transport
- Supports chain-of-custody documentation during clinical trials
FDA-approved tape does more than protect—it strengthens communications within distribution networks. When inspectors, manufacturers, and logistics teams see tamper-evident signals, they can act quickly to prevent risks before products reach patients.
FDA Security Labels: Product Authentication
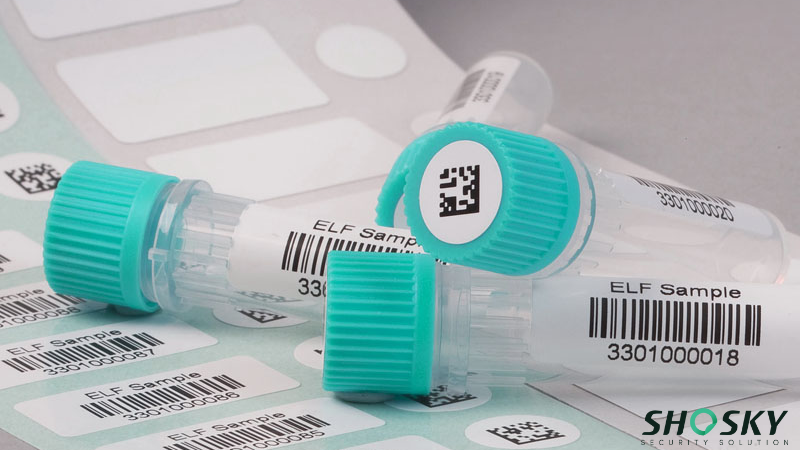
FDA Security Labels are types of security labels that enhance the security of pharmaceutical products by adding layers of authentication. They come with features like serialization, barcodes, and QR codes that allow inspectors and distributors to verify product origin and movement. This level of traceability is vital for stopping counterfeiting and ensuring compliance with FDA standards.
- Applied to pill bottles, vials, cartons, and shipping cases to confirm authenticity
- Can be integrated with digital platforms for real-time tracking
- Available with specialty adhesives suitable for different surfaces
- Offers options for variable printing, including lot numbers and expiration dates
FDA-compliant labels do more than meet technical requirements—they help guarantee public health by making it harder for falsified drugs to reach the market. They also give regulators and pharmacies confidence that the product being dispensed is authentic and safe for sale.
Core Features of FDA-Compliant Security Tapes and Labels for Pharma
In the pharma industry, FDA security tape and labels help maintain FDA compliance while protecting drug containers from tampering at every stage. The following sections break down its core features in detail:
1. Tamper-Evident Design
The FDA security tape and label are engineered with tamper-evident features that make any interference clearly visible. If someone tries to open the package, they may split, break apart, or display a “VOID” message.
In some cases, they leave permanent residue patterns that cannot be removed without damaging the packaging. These indicators provide undeniable proof of tampering.
Tamper-evident design is a critical element of pharmaceutical security packaging. Visible signs of tampering protect high-value pharma shipments, safeguard public health, and also support FDA compliance.
By maintaining the integrity of drug containers during shipping, storage, and distribution, manufacturers can reduce the risk of compromised products and build trust with consumers and regulators.
2. Material Safety and Non-Toxic Adhesives
FDA-approved security tape and labels use specifically designed adhesive formulations that meet strict safety standards. These adhesives are safe for direct or indirect contact with pharmaceutical products. This is important to avoid harmful chemical migration into the drug or its packaging and to compromise their effectiveness.
Materials used in either FDA security tape or FDA security label are tested to ensure they do not affect the shelf life of medicines. Using FDA-compliant adhesive solutions protects both the product and the end consumer. It also helps manufacturers meet regulatory requirements for packaging safety.
3. High Adhesion and Packaging Integrity
FDA-compliant tapes and labels are built to create secure seals across a wide range of packaging types, including cartons, bottles, blister packs, and even metal or paper-based containers. Their strong adhesion keeps packaging intact during storage and distribution, helping prevent compliance issues caused by packaging failure.
Both solutions maintain effectiveness under different environmental conditions, whether products are shipped in cold chain settings or stored at room temperature. This reliability safeguards packaging integrity, lowers the chance of tampering or accidental opening, and supports trust across the supply chain.
4. Serialization and Track-and-Trace
Security tapes and labels with FDA compliance can include serialization features like barcodes, QR codes, or RFID tags. These identifying characteristics help track and trace drug packages through the supply chain. This supports the Drug Supply Chain Security Act (DSCSA).
Serialization allows manufacturers and regulators to verify the authenticity of each package. It also helps identify and isolate compromised or counterfeit products. This strengthens protection against theft and diversion.
5. Visual and Covert Security Features
Some FDA security tape and labels options are designed with advanced visual elements such as color-shifting inks, microtext, or holograms. These features make tampering easier to spot and allow inspectors to authenticate packaging quickly. Covert security elements, hidden from view, add another layer of verification that can be confirmed during an inspection or review process.
By combining visible and covert elements, manufacturers can better protect drug containers from counterfeiting and strengthen pharmaceutical security. The data gathered from these security checks can help identify risks in the supply chain and improve productivity by reducing the need for rework or product recalls.
FDA Compliance Security Tape & Labels vs. Standard Security Tape & Labels
Security tapes and labels are not all the same. FDA-compliant versions are specifically designed for pharmaceutical use, meeting strict requirements for safety, adhesion, and tamper evidence. Standard options, on the other hand, may not provide the same level of protection or compliance. Here is a quick comparison:
| Feature | FDA-Approved Security Tape & Labels | Standard Security Tape & Labels |
|---|---|---|
| Material Safety | Uses FDA-approved, non-toxic adhesives | Adhesives may not be tested for pharma safety |
| Tamper-Evident Features | Breaks, void messages, residue patterns | Basic tamper evidence, often less reliable |
| Adhesion and Seal Strength | High adhesion for various packaging materials | Variable adhesion may fail under stress |
| Support for Serialization | Can integrate barcodes, QR codes, and RFID | Rarely supports track-and-trace features |
| Suitable for Cold Chain | Tested for temperature and humidity variations | May lose adhesion in extreme conditions |
| Risk of Chemical Migration | Low, tested for safety | Higher risk due to untested materials |
Using non-compliant tape or labels creates risks of undetected tampering, contamination, and counterfeiting. It can also cause failures during FDA audits, leading to penalties or product recalls. By investing in FDA-approved security tape and labels, manufacturers strengthen packaging integrity, improve communication within the supply chain, and protect both patients and public health.
Applications of FDA Security Tape & Labels in the Pharmaceutical Supply Chain
FDA-approved security tape and labels are used in many applications, from protecting individual bottles and cartons to securing shipping pallets and clinical trial materials. The following examples show in detail where this tape is most commonly applied.
Packaging Bottles, Blister Packs, and Cartons
FDA-approved security tapes and labels are essential for securing pharmaceutical bottles, blister packs, and cartons. They act as security tools that provide clear, visible evidence that the product is intact and hasn’t been tampered with. This is especially important for fragile items like crushable glass ampules and products with capsule sealing technologies.
Many pharmaceutical manufacturers include a labeling statement or other identifying characteristic on the tape itself. This supports both product authentication and regulatory compliance. Using tamper-evident packaging in this way ensures the integrity of the medicine from production through delivery.
Research indicates that implementing tamper-evident packaging, such as FDA-approved security tape and labels, on packaging bottles, blister packs, and cartons can reduce incidents of product tampering. A report from the National Institutes of Health (NIH) also highlights that secure packaging helps prevent theft and counterfeiting, which costs the industry billions each year.
Sealing Shipping Boxes and Pallets
FDA-approved security tapes and labels are essential for keeping shipping boxes and pallets secure during distribution. They maintain seal integrity and provide clear tamper-evident indicators that reveal any attempt at unauthorized access. This level of visibility is especially important in pharmaceutical supply chains where product safety cannot be compromised.
Some solutions go further by integrating unique identifiers such as barcodes, QR codes, or serialized numbers. These features support traceability, FDA compliance, and audit readiness by creating a verifiable record of each package throughout its journey.
The strength of FDA-approved tapes and labels also reduces the risks of theft or contamination. Their durability allows them to hold up against temperature fluctuations, warehouse conditions, and rough handling, making them a dependable choice for securing high-value pharmaceutical shipments.
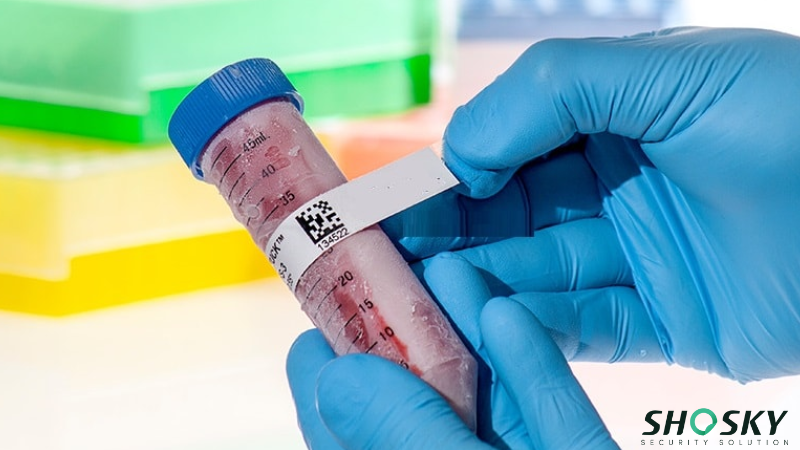
Securing Clinical Trial Materials
Clinical trial materials require tamper-evident packaging solutions to ensure the integrity of the study and patient safety. FDA-approved security tape and labels are commonly used to seal clinical trial kits and containers. Their tamper-evident design provides visible evidence of interference, which is crucial for maintaining the chain of custody.
These tamper-evident solutions help protect trial products sealed in capsule packaging, blister packs, and even fragile glass ampules. They also prevent tampering with sensitive items like ammonia inhalants or aerosol products used in clinical settings.
Using FDA-compliant solutions safe for indirect food contact helps meet strict regulatory requirements while maintaining product integrity during storage, shipping, and handling. Clear labeling statements and serialization features—such as barcodes or QR codes—also support product traceability and allow quick verification by auditors, regulatory agencies, and authorized parties.
Use in Cold Chain Logistics
Cold chain logistics demand packaging that maintains security under extreme temperatures and humidity. FDA-approved security tape and labels are designed to perform reliably in these conditions, ensuring a strong seal on packages that may contain temperature-sensitive drugs.
These solutions provide visible evidence if tampering occurs, even in cold storage. It is effective on containers holding compressed medical oxygen, aerosol products, and other sensitive pharmaceuticals that require stable conditions.
Using FDA-compliant tamper tape in cold chain logistics also helps maintain the tamper-resistant package throughout distribution. This supports drug safety, preserves shelf life, and meets regulatory compliance in challenging environments.
Best Practices for Implementing Tamper-Evident Tapes & Labels in Pharma Operations for Public Health
Proper employee training is essential when implementing tamper-evident solutions (whether it is tape or a label) in pharmaceutical operations. Staff should understand how to apply the tape correctly and recognize signs of tampering. Integrating these steps into standard operating procedures (SOPs) helps maintain consistency and reduce errors during packaging.
Inspection points should be built into every stage of handling to confirm seals and labels remain intact. Quick detection of tampering prevents counterfeit or compromised products from entering the supply chain and strengthens compliance with FDA regulations.
Maintaining accurate records of tape and label specifications, inspection outcomes, and any process changes is equally important. Clear communication across packaging teams and ongoing awareness ensure these solutions work effectively.
Following these best practices protects patients, secures distribution, and reinforces trust in pharmaceutical products.
FAQs
How is FDA-approved tamper tape tested for safety?
It’s tested for safe adhesives, chemical migration, durability, and packaging compatibility.
Does an FDA-approved security label need to meet indirect food contact requirements?
Yes. This ensures that adhesives and materials will not contaminate products if incidental contact occurs.
What’s the difference between FDA approval and FDA compliance?
FDA approval means formal authorization; compliance means following FDA standards in production and packaging.
Conclusion
FDA security solutions like tapes and labels are a small investment with a big impact. Their tamper-evident features protect pharmaceutical products, support compliance, and maintain packaging integrity. And choosing the right solution and using it properly can save costs linked to recalls or breaches. Combined with serialization and tracking, these solutions help with supply chain security and build lasting trust with customers!
Secure Your Packaging with FDA Security Tape from Shosky Security
Shosky Security offers FDA security solutions, including FDA tapes and FDA labels designed to protect your pharmaceutical products. So, don’t compromise on safety or compliance! Contact us today and find the right FDA-approved security solutions for your needs!